OUR SERVICES
Contract Manufacturing Services
Aseptic Fill-Finish
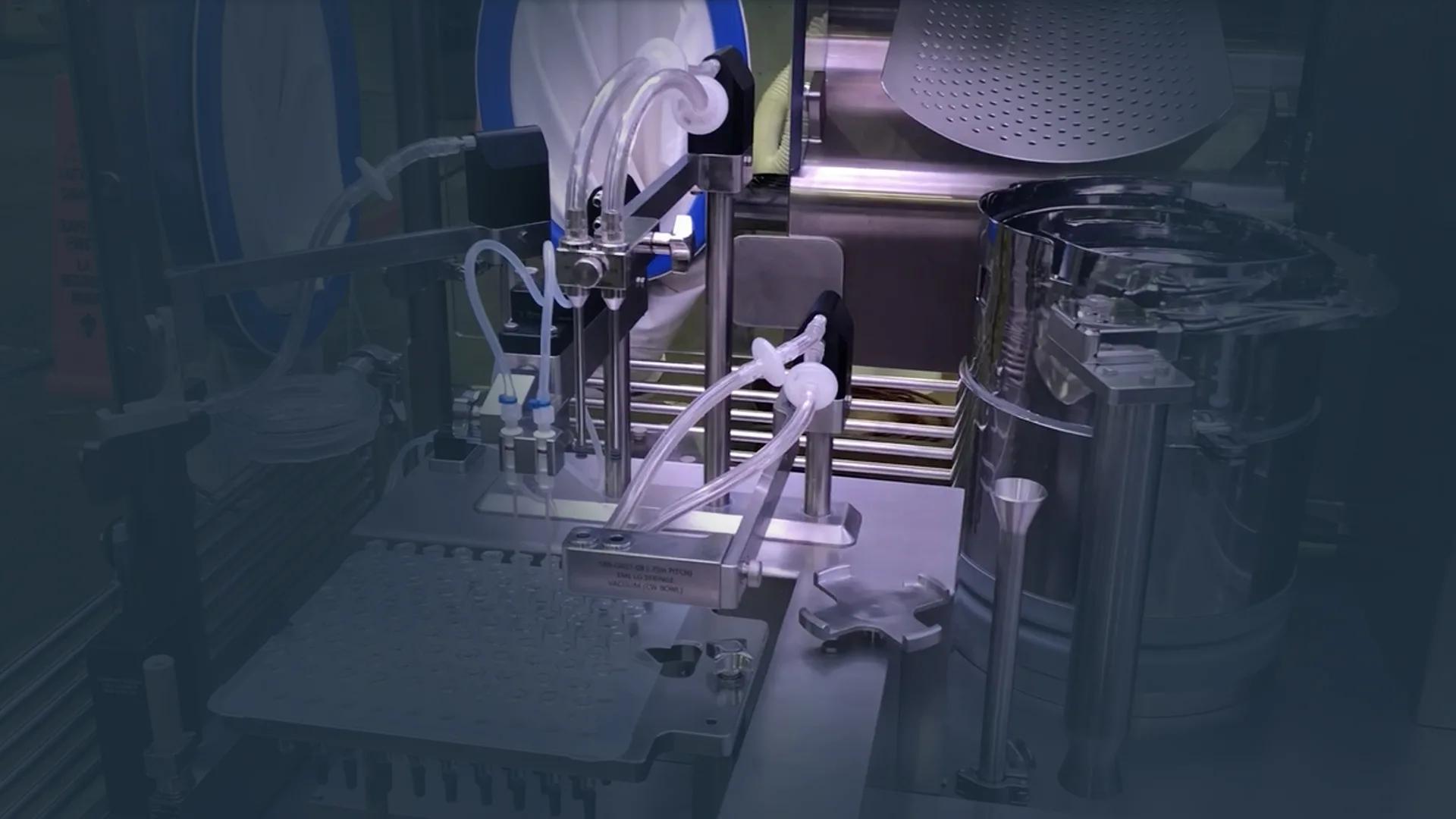
AscentX Medical is a Contract Development and Manufacturing Organization (CDMO) currently offering aseptic fill and finish services for non-human-use applications. These services are conducted in an ISO-certified cleanroom facility, operated under internal procedures aligned with current Good Manufacturing Practices (cGMPs).
While AscentX does not yet manufacture products for human clinical or commercial use, we are actively preparing for future expansion. Upon successful inspection and approval by the California Department of Public Health (CDPH) and the U.S. Food and Drug Administration (FDA), our facility will transition to full cGMP-compliant operations supporting human-use products.
Our current capabilities include formulation, syringe and vial filling, packaging, and the handling of a wide range of non-human-use pharmaceutical and medical device components. These services support process development, documentation preparation, preclinical animal studies, feasibility testing, formulation work, and regulatory requirements.
We welcome early-phase collaboration to help advance your project. If there is a service you need not listed here, please contact us to discuss your unique project needs.
Research and Development Support
AscentX Medical is committed to driving innovation through rigorous research and development. Our R&D support services encompass clinical trial management, process development, and regulatory assistance. Our team of experienced scientists can help with formulation, method, and process development, ensuring that your projects are supported at every stage, from initial concept to regulatory approval.
Quality Assurance and Compliance
Quality is at the core of AscentX Medical’s operations. Our comprehensive quality assurance and compliance services ensure that all products and processes adhere to stringent regulatory standards. From initial development to final production, we maintain the highest levels of quality and safety. Our commitment to excellence is reflected in our rigorous quality control measures and continuous improvement initiatives. Additionally, we offer assistance in developing Standard Operating Procedures (SOPs) and production batch record forms, ensuring that your processes are well-documented and compliant with industry standards.
Facilities
Our state-of-the-art facilities are designed to support a wide range of contract manufacturing and development needs. Equipped with ISO Class 5 sterile suites and ISO Class 8 clean rooms, we specialize in aseptic fill and finish processes, including syringe filling and vial filling. Our facilities are monitored 24/7 for security and environmental conditions, ensuring the integrity and quality of all products. Additionally, we offer qualified storage areas for USP refrigerated and controlled room temperature conditions, as well as white glove-controlled shipping services to ensure products reach clinical sites in pristine condition.
Explore how AscentX Medical’s advanced capabilities and commitment to quality can support your unique projects. If there is a service you need not listed here, please contact us to discuss your specific requirements.
Technology
Injectable Regenerative Therapy
Advanced Autologous Tissue Engineering
Literature