As per DelveInsight analysis in the stress urinary incontinence devices market, the growing demand for stress urinary incontinence is predominantly attributed to the increasing prevalence of stress urinary incontinence amongst women across the globe. Another prominent factor is the rising incidence of diabetes mellitus, obesity, and Parkinson’s disease, acting as risk factors for the development of stress urinary incontinence amongst the population. In addition to the factors mentioned above, the development of new and emerging stress urinary incontinence devices is also expected to play a key role in establishing an upward growth trend in the stress urinary incontinence devices market during the forecast period from 2022 to 2027.
August 30, 2022 13:00 ET| Source: DelveInsight Business Research LLP
New York, USA, Aug. 30, 2022 (GLOBE NEWSWIRE) — The Global Stress Urinary Incontinence Devices Market to Surpass USD 1 Billion Mark by 2027, Assesses DelveInsight
As per DelveInsight analysis in the stress urinary incontinence devices market, the growing demand for stress urinary incontinence is predominantly attributed to the increasing prevalence of stress urinary incontinence amongst women across the globe. Another prominent factor is the rising incidence of diabetes mellitus, obesity, and Parkinson’s disease, acting as risk factors for the development of stress urinary incontinence amongst the population. In addition to the factors mentioned above, the development of new and emerging stress urinary incontinence devices is also expected to play a key role in establishing an upward growth trend in the stress urinary incontinence devices market during the forecast period from 2022 to 2027.
DelveInsight’s Stress Urinary Incontinence Devices Market Insights report provides the current and forecast market, forthcoming device innovation, individual leading companies’ market shares, challenges, stress urinary incontinence devices market drivers, barriers, and trends, and key stress urinary incontinence devices companies in the market.
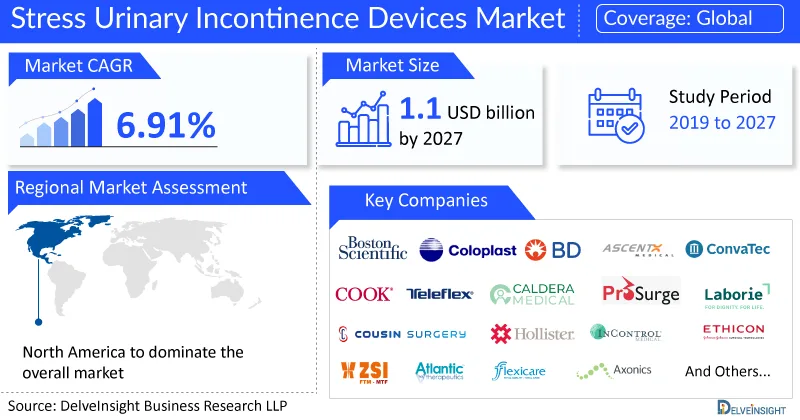
Key Takeaways from the Stress Urinary Incontinence Devices Market Report
- As per DelveInsight estimates, North America is anticipated to dominate the global stress urinary incontinence devices market during the forecast period.
- Notable stress urinary incontinence devices companies such as Boston Scientific Corporation, AscentX Medical Inc., Coloplast Corporation, Becton, Dickinson and Company, ConvaTec Group PLC, Ethicon US, LLC (Johnson & Johnson), Cook Medical Inc., Teleflex Incorporated, Caldera Medical Inc., Prosurg, Inc., Laborie, InControl Medical, Cousin Surgery, Hollister Incorporated, ZSI Surgical Implants, Atlantic Therapeutics, Flexicare (Group) Limited, A.M.I. GmbH, Axonics, Inc., Betatech Medical, and several others are currently operating in the stress urinary incontinence devices market.
- In March 2022, Pelvic floor muscle training using a motion-based digital intravaginal device significantly improved symptoms for women with stress urinary incontinence (SUI) compared with a home training program, a virtually conducted randomized controlled trial showed.
- On August 21, 2020, the Food and Drug Administration granted clearance for Flyte™ (Pelvital), a noninvasive, intravaginal home-use device intended for the treatment of stress urinary incontinence (SUI) in patients aged ≥21 years.
- On February 05, 2020, Contura, a medical device company that helps in developing, manufacturing, and commercializing products using its unique patented hydrogel technology, received Food and Drug Administration approval for its Bulkamid hydrogel for treating stress urinary incontinence (SUI) in adult women with SUI or stress predominant mixed incontinence.
- Thus, owing to such developments in the market, rapid growth will be observed in the stress urinary incontinence devices market during the forecast period.
To read more about the latest highlights related to the stress urinary incontinence devices market, get a snapshot of the key highlights entailed in the Global Stress Urinary Incontinence Devices Market Report.
Stress Urinary Incontinence Devices
Stress Urinary Incontinence (SUI) occurs when urine leaks out due to sudden pressure on the bladder and urethra, causing the sphincter muscles to open briefly. Pressure may be caused by sudden forceful activities in cases of mild SUI; however, in cases of severe SUI, the individual tends to leak even from less forceful activities. It is a prevalent bladder problem in women and may occur less frequently in men. SUI can be treated with medical devices that aid in developing and strengthening the pelvic floor muscles for the treatment of stress urinary incontinence. Electrical muscle stimulation is included in the devices, which aids in providing an effective, safe, and non-invasive form of treatment.
Stress Urinary Incontinence Devices Market Insights
The global stress urinary incontinence devices market is studied geographically for North America, Europe, Asia-Pacific, and the Rest of the World. North America is expected to amass a significant revenue share in the global stress urinary incontinence devices market during the forecast period, with the largest market share. The rising prevalence of urological disorders, the increased development of advanced urinary incontinence devices, and the rising demand for minimally invasive operations are some of the factors driving the growth of the North American stress urinary incontinence devices market. The growing geriatric population, which is more prone to the development of urological problems, is also one of the factors contributing to the region’s growing demand for stress urinary incontinence devices. On the other hand, the European market will challenge North America’s dominance.
The growing prevalence of the senior population in the United States is one of the key factors driving the growth of the North American stress urinary incontinence devices market. Furthermore, an increase in the prevalence of urinary incontinence in Canada will increase the demand for stress urinary incontinence devices, ultimately leading to the rise in the overall stress urinary incontinence devices market growth.
To know more about why North America is leading the market growth in the stress urinary incontinence devices market, get a snapshot of the Stress Urinary Incontinence Devices Market Trends.
Stress Urinary Incontinence Devices Market Dynamics
The global stress urinary incontinence market is expected to grow significantly due to increased demand for stress urinary incontinence devices due to increased emphasis on developing various robust treatments and other management strategies for reducing the burden of stress urinary incontinence among the population. Another key factor driving the growth of the stress urinary incontinence devices market is the increasing development of safe and efficient electrical stimulation devices. Furthermore, rising government investment and expenditure for effectively managing stress urinary incontinence is propelling the stress urinary incontinence devices market forward.
Aside from the factors mentioned above, another key factor driving the growth of the stress urinary incontinence devices market is the rising demand for minimally invasive operations and the increasing number of stress urinary incontinent patient population shifting from traditional to newer and emerging stress urinary incontinence devices.
However, postoperative complications and a lack of awareness among the patient population about stress incontinence may be limiting factors in the growth of the stress urinary incontinence devices market.
Additionally, the stress urinary incontinence devices market experienced a brief period of market restraint due to the implementation of lockdown as necessary measures to break the chain of COVID-19 infection transmission. The imposition of the lockdown had a significant impact on supply chains, and production disruptions affected device production. During the COVID-19 crisis, many countries separated medical procedures based on the need for urgent medical care to streamline the workflow, and various elective medical procedures were suspended. This resulted in lower product demand for stress urinary incontinence devices than was seen at normal levels. However, the stress urinary incontinence devices market has improved with the resumption of activities across industries, including the healthcare sector.
Scope of the Stress Urinary Incontinence Devices Market Report
- Porter’s Five Forces Analysis, Product Profiles, Case Studies, KOL’s Views, Analyst’s View
- Coverage: Global
- Study Period: 2019–2027
- Market Segmentation By Product: Vaginal Pessaries, Sling Systems, Artificial Urinary Sphincters, Urethral Bulking Agent, Others
- Market Segmentation By Type: External Devices, Internal Devices
- Market Segmentation By Patient Type: Male, Female
- Market Segmentation By End User: Hospitals, Ambulatory Surgery Centers, Others
- Market Segmentation By Geography: North America, Europe, Asia-Pacific, and Rest of World
- Key Stress Urinary Incontinence Devices Companies: Boston Scientific Corporation, AscentX Medical Inc., Coloplast Corporation, Becton, Dickinson and Company, ConvaTec Group PLC, Ethicon US, LLC (Johnson & Johnson), Cook Medical Inc., Teleflex Incorporated, Caldera Medical Inc., Prosurg, Inc., Laborie, InControl Medical, Cousin Surgery, Hollister Incorporated, ZSI Surgical Implants, Atlantic Therapeutics, Flexicare (Group) Limited, A.M.I. GmbH, Axonics, Inc., Betatech Medical, among others
- Porter’s Five Forces Analysis, Product Profiles, Case Studies, KOL’s Views, Analyst’s View
DelveInsight Analysis: The stress urinary incontinence devices market size is expected to grow at a CAGR of 6.91% to reach USD 1.13 billion by 2027.
Which MedTech key players in the stress urinary incontinence devices market are set to emerge as the trendsetter explore @Stress Urinary Incontinence Devices Companies
Table of Contents
| 1 | Report Introduction |
| 2 | Executive summary |
| 3 | Regulatory and Patent Analysis |
| 4 | Key Factors Analysis |
| 5 | Porter’s Five Forces Analysis |
| 6 | COVID-19 Impact Analysis on Stress Urinary Incontinence Devices Market |
| 7 | Stress Urinary Incontinence Devices Market Layout |
| 8 | Global Company Share Analysis – Key 3-5 Companies |
| 9 | Stress Urinary Incontinence Devices Market Company and Product Profiles |
| 10 | Project Approach |
| 11 | About DelveInsight |
Interested in knowing the stress urinary incontinence devices market by 2027? Click to get a snapshot of the Stress Urinary Incontinence Devices Market Scenario
Related Reports
Stress Urinary Incontinence Market
Stress Urinary Incontinence Market Insight, Epidemiology, and Market Forecast, 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key stress urinary incontinence companies, including Adrian Gaspar, Gynamics LTD, Solace Therapeutics, Inc., among others.
Stress Urinary Incontinence Pipeline
Stress Urinary Incontinence Pipeline Insight, 2022 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key stress urinary incontinence companies including Adrian Gaspar, Gynamics LTD, Solace Therapeutics, Inc., among others.
Stress Urinary Incontinence Epidemiology
Stress Urinary Incontinence Epidemiology Forecast to 2032 report delivers an in-depth understanding of the disease, historical, and forecasted stress urinary incontinence epidemiology in the 7MM.
Urinary Incontinence Pipeline Insight, 2022 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key urinary incontinence companies including Ixaltis SA, Graminex LLC, KGK Science Inc., among others.
Urinary Incontinence Market Insight, Epidemiology, and Market Forecast, 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key urinary incontinence companies, including Ixaltis SA, Graminex LLC, KGK Science Inc., among others.
About DelveInsight
DelveInsight is a leading Business Consultant, and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance.
About AscentX Medical
AscentX Medical, Inc. is a San Diego, California-based, privately-held medical technology company with a wholly-owned European subsidiary, AscentX Medical Europe Ltd., incorporated in London, UK. The company is focused on the development of least-invasive, curative outpatient treatments to improve the quality of life of the growing aging population. The company was founded by the original team that invented ArteFill® – the first and only U.S. FDA-approved permanent injectable wrinkle and acne scar filler – to clinically advance its technology platform consisting of injectable polymethylmethacrylate (PMMA)-collagen implants for permanent soft tissue augmentation. AscentX Medical’s primary focus is on the clinical testing of G125, specifically developed as an outpatient, cost-effective, and curative treatment for GERD. For more information, visit ascentxmedical.com.
About the SME Instrument
With approximately €3 billion in funding over 2014-2020 under the EU’s Horizon 2020 initiative, the SME (small to medium enterprise) Instrument supports high-potential innovators with equity-free and non-dilutive funding during a crucial point in their development – from the early stages to market introduction. Its focus is on fostering business opportunities that will shape new markets and generate jobs, economic growth, and higher standards of living. Companies applying for the SME Instrument are assessed exclusively on their business and innovation merit, and are required to demonstrate that there is a market for their innovation as well as potential customers willing to pay for it. The SME Instrument Phase I grant is awarded to just 8% of more than 3,000 applicants, with less than 5% percent of all recipients advancing into Phase II. For more information, visit ec.europa.eu.
CORPORATE MEDIA CONTACT:
Sara Adams
PR for AscentX Medical, Inc.
619.647.1898 / sadams@ascentxmedical.com
SOURCE AscentX Medical, Inc.

